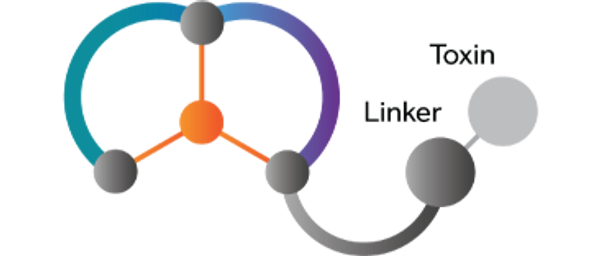
LONDON, U.K., CAMBRIDGE, U.K, and BOSTON, Mass., Feb. 13, 2018 — Cancer Research UK and Bicycle Therapeutics, a biotechnology company pioneering a new class of therapeutics based on its proprietary bicyclic peptide (Bicycle®) product platform, announced today that the first patient has been dosed in their Phase I/IIa trial evaluating BT1718 in patients with advanced solid tumours. BT1718 is a first-in-class Bicycle Toxin Conjugate being developed by Bicycle Therapeutics that targets Membrane Type 1 Matrix Metalloproteinase (MT1-MMP/MMP-14), which has been shown to be highly expressed in solid tumours.
“The initiation of this study is a landmark event for the company and for our technology,” said Maria Koehler, M.D., Ph.D., Chief Medical Officer of Bicycle Therapeutics. “BT1718 is the first clinical candidate from our pipeline of Bicycles, a brand-new class of chemically synthesized medicines. We believe that Bicycles, because of their small size and exquisite selectivity, could provide meaningful efficacy to patients suffering from cancer and avoid the toxicities associated with other classes of highly potent anti-cancer drugs. We are delighted to be exploring its potential in collaboration with Cancer Research UK.”
The approximately 120-patient Phase I/IIa trial is designed to evaluate the safety and preliminary efficacy of BT1718 in patients with high expression of tumour MT1, as measured by a proprietary MT1 immunochemistry assay. Following a rapid dose escalation phase, the Phase I/IIa trial will evaluate two schedules of BT1718.
“We are excited to initiate this clinical study of BT1718, the first in a promising new class of potent anticancer agents with strong potential to deliver a meaningful therapeutic impact,” said Dr. Udai Banaji, Principal Investigator for the Phase I/IIa trial. “Our team is eager to evaluate this important new therapeutic in patients with advanced solid tumours.”
Dr. Nigel Blackburn, Cancer Research UK’s director of drug development, said: “BT1718 is a potentially transformative treatment that has shown great promise in preclinical studies, and trials like this are a big step towards helping more patients survive their cancer. We urgently need new, safe and effective therapies for patients with hard to treat cancers such as non-small cell lung cancer and triple negative breast cancer that this drug will tested on. Supporting this type of innovative clinical research is a key priority for Cancer Research UK.”
ABOUT BT1718
BT1718 is a first-in-class Bicycle Toxin Conjugate being developed by Bicycle Therapeutics that targets Membrane Type 1 Matrix Metalloproteinase (MT1-MMP), also known as MMP-14, which has an established role in cell invasion and metastasis, is linked to poor outcomes and is over expressed in many solid tumours. BT1718 has demonstrated promising target-dependent efficacy in preclinical models, including both cell- and patient-derived xenografts that are resistant to treatment with standards of care. In addition, it shows only a subset of the toxicities typically associated with other highly potent cancer treatments.
Cancer Research UK’s Centre for Drug Development (CDD) is sponsoring a Phase I/IIa study of BT1718. The trial is co-managed by Cancer Research UK and Bicycle Therapeutics. Under the terms of the agreement, Bicycle retains the right to further advance the BT1718 program, at which point an undisclosed payment split between cash and equity, success based milestones and royalty payments would be made to Cancer Research UK.
ABOUT BICYCLE THERAPEUTICS
Bicycle Therapeutics is developing a unique class of chemically synthesized medicines based on its proprietary bicyclic peptide (Bicycle®) product platform to address therapeutic needs unreachable with existing treatment modalities. Bicycle’s internal focus is in oncology, where the company is developing targeted cytotoxics (Bicycle Toxin Conjugates), targeted innate immune activators and T-cell modulators for cancers of high unmet medical need. Bicycles’ small size and exquisite targeting delivers rapid tumour penetration and retention while clearance rates and routes of elimination can be tuned to minimise exposure of healthy tissue and bystander toxicities. The company’s lead program, BT718, is being evaluated in a Phase I/IIa trial in collaboration with Cancer Research, UK. The company’s unique intellectual property is based on the work initiated at the MRC Laboratory of Molecular Biology in Cambridge, U.K., by the scientific founders of the company, Sir Gregory Winter and Professor Christian Heinis. Bicycle has its headquarters in Cambridge, U.K., with many key functions and members of its leadership team located in the biotech hub of Boston, Mass. For more information, visit www.bicycletherapeutics.com or follow us on Twitter at @Bicycle_tx.
ABOUT CANCER RESEARCH UK’S COMMERCIAL PARTNERSHIPS TEAM
Cancer Research UK is the world’s leading cancer charity dedicated to saving lives through research. Our specialist Commercial Partnerships Team work closely with leading international cancer scientists and their institutes to protect intellectual property arising from their research and to establish links with commercial partners. The team develop promising ideas into successful cancer therapeutics, software, devices, diagnostics and enabling technologies. This helps to accelerate progress in exciting new discoveries in cancer research and bring new treatments to patients sooner. http://commercial.cancerresearchuk.org/
Cancer Research UK’s commercial activity operates through Cancer Research Technology Ltd. (CRT), a wholly owned subsidiary of Cancer Research UK. It is the legal entity which pursues drug discovery research in themed alliance partnerships and delivers varied commercial partnering arrangements.
ABOUT CANCER RESEARCH UK’S CENTRE FOR DRUG DEVELOPMENT
Cancer Research UK has an impressive record of developing novel treatments for cancer. The Cancer Research UK Centre for Drug Development, formerly the Drug Development Office, has been pioneering the development of new cancer treatments for 25 years, taking over 140 potential new anti-cancer agents into clinical trials in patients. It currently has a portfolio of around 30 new anti-cancer agents in preclinical development, Phase I or early Phase II clinical trials. Six of these new agents have made it to market including temozolomide for brain cancer, abiraterone for prostate cancer and rucaparib for ovarian cancer. Two other drugs are in late development Phase III trials. This rate of success is comparable to that of any pharmaceutical company.
ABOUT CANCER RESEARCH UK
- Cancer Research UK is the world’s leading cancer charity dedicated to saving lives through research.
- Cancer Research UK’s pioneering work into the prevention, diagnosis and treatment of cancer has helped save millions of lives.
- Cancer Research UK receives no funding from the UK government for its life-saving research. Every step it makes towards beating cancer relies on vital donations from the public.
- Cancer Research UK has been at the heart of the progress that has already seen survival in the UK double in the last 40 years.
- Today, 2 in 4 people survive their cancer for at least 10 years. Cancer Research UK’s ambition is to accelerate progress so that by 2034, 3 in 4 people will survive their cancer for at least 10 years.
- Cancer Research UK supports research into all aspects of cancer through the work of over 4,000 scientists, doctors and nurses.
- Together with its partners and supporters, Cancer Research UK’s vision is to bring forward the day when all cancers are cured.
For further information about Cancer Research UK’s work or to find out how to support the charity, please call 0300 123 1022 or visit www.cancerresearchuk.org. Follow us on Twitter and Facebook.